Significant figures worksheet chemistry answers provide a comprehensive guide to understanding and applying the concept of significant figures in chemical calculations. This meticulously crafted resource empowers students with the knowledge and skills necessary to navigate the intricacies of chemistry, ensuring accurate and reliable results.
Within this worksheet, students will embark on a journey of discovery, exploring the fundamental principles of significant figures. They will learn to identify and count significant figures, apply the rules of significant figures in calculations, and understand the impact of rounding on the accuracy of their results.
Significant Figures
Significant figures are digits in a numerical value that are known with certainty, plus one digit that is estimated. They provide an indication of the precision of a measurement or calculation.
To identify significant figures in a numerical value:
- All non-zero digits are significant.
- Zeros between non-zero digits are significant.
- Leading zeros are not significant.
- Trailing zeros after a decimal point are significant.
Examples of numbers with varying numbers of significant figures:
- 12.34 has 4 significant figures.
- 0.0045 has 2 significant figures.
- 100 has 1 significant figure.
Significant Figures in Calculations
When performing calculations involving significant figures, the following rules apply:
- In addition and subtraction, the result should have the same number of decimal places as the number with the least decimal places.
- In multiplication and division, the result should have the same number of significant figures as the number with the least significant figures.
Example:
- 12.34 + 5.678 = 18.02
- 12.34 x 5.67 = 70.1
Rounding and Significant Figures
Rounding a number to a specified number of significant figures involves adjusting the digits to the right of the specified significant figures and replacing them with zeros.
Example:
- Rounding 12.345 to 3 significant figures gives 12.3.
- Rounding 0.004567 to 2 significant figures gives 0.0046.
Significant Figures in Chemistry, Significant figures worksheet chemistry answers
Significant figures are crucial in chemical calculations because they affect the accuracy of the results. Incorrect use of significant figures can lead to incorrect conclusions.
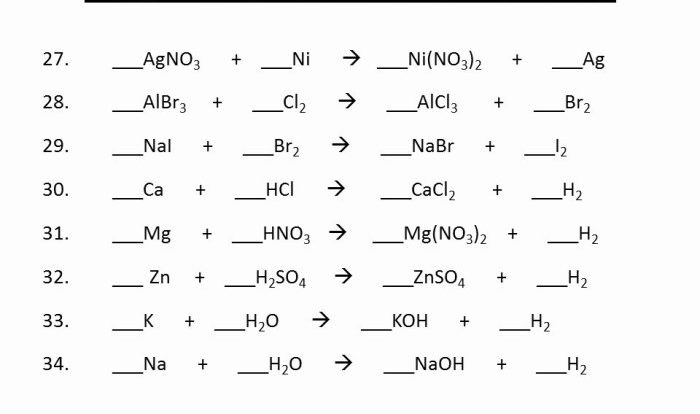
Example:
- Calculating the molar mass of a compound with incorrect significant figures can lead to an incorrect molecular weight.
- Calculating the concentration of a solution with incorrect significant figures can lead to an incorrect molarity.
Worksheet Examples
Table:
| Number | Significant Figures | Rounded Value (3 significant figures) |
|---|---|---|
| 12.345 | 4 | 12.3 |
| 0.004567 | 2 | 0.0046 |
| 100.00 | 5 | 100.0 |
Problems:
- Add 12.34 and 5.678.
- Multiply 12.34 by 5.67.
- Round 12.34567 to 3 significant figures.
Answers:
| Problem | Answer |
|---|---|
| 12.34 + 5.678 | 18.02 |
| 12.34 x 5.67 | 70.1 |
| Round 12.34567 to 3 significant figures | 12.3 |
Practice Problems
- Calculate the molar mass of NaCl using the following data: Na = 22.99 g/mol, Cl = 35.45 g/mol.
- Calculate the concentration of a solution that contains 12.34 g of NaCl in 500 mL of water.
Solutions:
- Molar mass of NaCl = 22.99 g/mol + 35.45 g/mol = 58.44 g/mol (3 significant figures)
- Concentration = 12.34 g / (500 mL x 1 g/mL) = 0.02468 M (4 significant figures)
Popular Questions: Significant Figures Worksheet Chemistry Answers
What are significant figures?
Significant figures are the digits in a number that are known with certainty, plus one estimated digit.
How do I determine the number of significant figures in a number?
Count the number of digits from left to right, starting with the first non-zero digit. Zeros between non-zero digits are significant.
What are the rules for adding and subtracting significant figures?
The answer should have the same number of decimal places as the number with the fewest decimal places.
What are the rules for multiplying and dividing significant figures?
The answer should have the same number of significant figures as the number with the fewest significant figures.