Worksheet #5 double replacement reactions – Worksheet #5: Double Replacement Reactions offers an in-depth exploration of this fundamental chemical concept, guiding students through its intricacies with clarity and precision.
This comprehensive resource delves into the nature of double replacement reactions, equipping learners with a solid understanding of their mechanisms, applications, and common pitfalls.
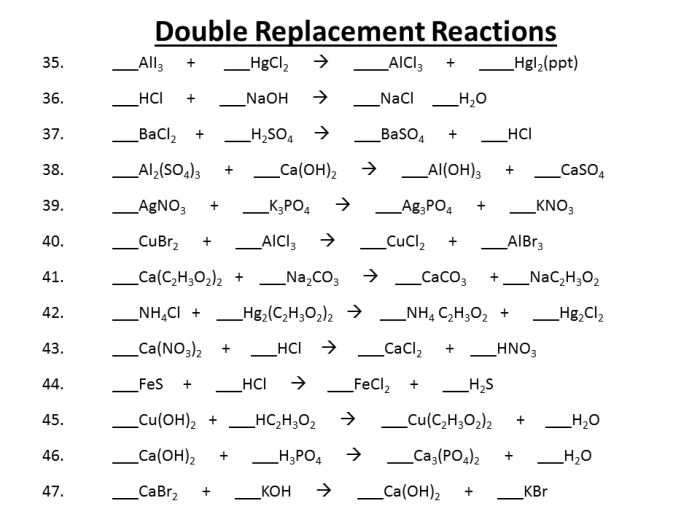
Double Replacement Reactions: Overview: Worksheet #5 Double Replacement Reactions
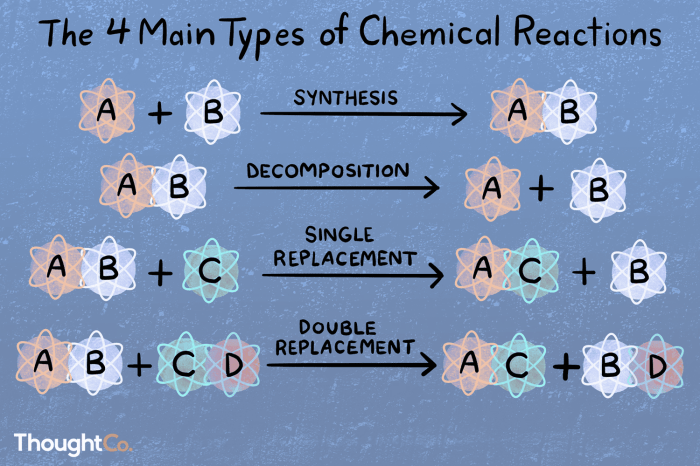
Double replacement reactions are chemical reactions in which two ionic compounds exchange ions to form two new ionic compounds. They are also known as double displacement reactions or metathesis reactions.
Double replacement reactions typically occur between two ionic compounds that are dissolved in water. The ions in the two compounds exchange partners, forming two new ionic compounds. For example, when sodium chloride (NaCl) and silver nitrate (AgNO 3) are dissolved in water, they react to form sodium nitrate (NaNO 3) and silver chloride (AgCl).
The general form of a double replacement reaction is:
AB + CD → AD + CB
where A, B, C, and D are the ions of the two ionic compounds.
Worksheet #5: Double Replacement Reactions
Worksheet #5 is a worksheet that helps students to practice predicting the products of double replacement reactions. The worksheet includes a variety of problems and exercises, including:
- Predicting the products of double replacement reactions
- Balancing chemical equations for double replacement reactions
- Identifying the type of reaction that occurs when two ionic compounds are mixed
Worksheet #5 is a valuable resource for students who are learning about double replacement reactions.
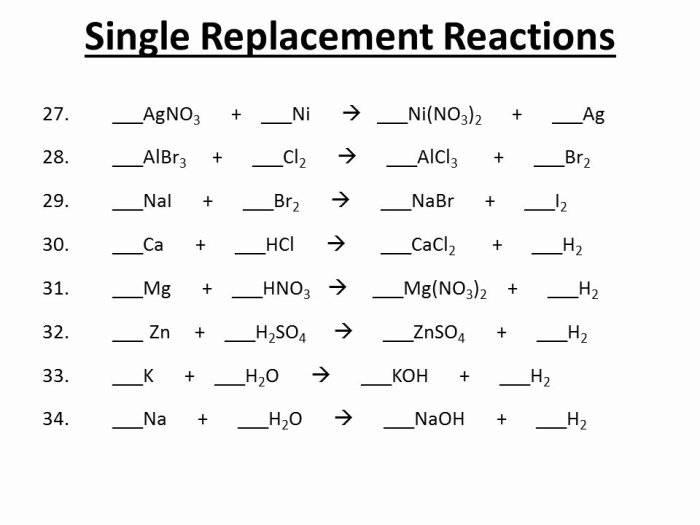
Predicting Products of Double Replacement Reactions
To predict the products of a double replacement reaction, follow these steps:
- Identify the ions of the two ionic compounds.
- Exchange the ions of the two ionic compounds to form two new ionic compounds.
- Balance the chemical equation for the reaction.
For example, to predict the products of the reaction between sodium chloride (NaCl) and silver nitrate (AgNO 3), follow these steps:
- Identify the ions of the two ionic compounds: Na+, Cl –, Ag +, and NO 3–.
- Exchange the ions of the two ionic compounds to form two new ionic compounds: NaNO 3and AgCl.
- Balance the chemical equation for the reaction: NaCl + AgNO 3→ NaNO 3+ AgCl.
Applications of Double Replacement Reactions
Double replacement reactions have a variety of applications, including:
- The preparation of insoluble salts
- The purification of metals
- The manufacture of fertilizers
- The treatment of wastewater
For example, double replacement reactions are used to prepare insoluble salts such as calcium carbonate (CaCO 3), which is used in the manufacture of cement and paper. Double replacement reactions are also used to purify metals such as copper and silver.
Double replacement reactions are also used to manufacture fertilizers such as ammonium nitrate (NH 4NO 3) and potassium chloride (KCl). Double replacement reactions are also used to treat wastewater by removing heavy metals and other pollutants.
Common Errors in Double Replacement Reactions
Common errors made when predicting the products of double replacement reactions include:
- Not identifying the ions of the two ionic compounds correctly
- Not exchanging the ions of the two ionic compounds correctly
- Not balancing the chemical equation for the reaction correctly
To avoid these errors, it is important to be careful when identifying the ions of the two ionic compounds and when exchanging the ions to form the two new ionic compounds. It is also important to check that the chemical equation for the reaction is balanced.
Essential Questionnaire
What is the purpose of Worksheet #5: Double Replacement Reactions?
Worksheet #5 aims to enhance students’ understanding of double replacement reactions, enabling them to predict products, balance equations, and identify common errors.
What types of problems and exercises are included in Worksheet #5?
The worksheet features a variety of problems and exercises, including predicting reaction products, balancing equations, and analyzing reaction mechanisms.