A chloro 2 6 dimethylacetanilide, an organic compound with a unique molecular structure and diverse applications, has garnered significant attention in the chemical industry. Its versatility, stability, and reactivity make it a valuable intermediate and additive in various fields. This comprehensive overview delves into the properties, synthesis, applications, safety considerations, market analysis, and related aspects of a chloro 2 6 dimethylacetanilide, providing a thorough understanding of this essential chemical.
With its distinctive molecular formula and chemical structure, a chloro 2 6 dimethylacetanilide exhibits intriguing physical and chemical properties. Its stability and reactivity characteristics contribute to its diverse applications, making it a versatile compound in various industries.
Chemical Structure and Properties
Chloro 2,6-dimethylacetanilide, also known as 4-chloro-2,6-dimethylacetanilide, is an organic compound with the molecular formula C 9H 12ClNO. It belongs to the class of compounds known as acetanilides, which are derivatives of acetanilide (N-phenylacetamide).
Chloro 2,6-dimethylacetanilide is a white or colorless crystalline solid with a melting point of 140-142 °C and a boiling point of 302-304 °C. It is slightly soluble in water and soluble in organic solvents such as ethanol, ether, and chloroform.
Physical Properties
Chloro 2,6-dimethylacetanilide is a white or colorless crystalline solid with a density of 1.22 g/cm 3. It has a melting point of 140-142 °C and a boiling point of 302-304 °C. It is slightly soluble in water and soluble in organic solvents such as ethanol, ether, and chloroform.
Chemical Properties
Chloro 2,6-dimethylacetanilide is a weak base and can react with acids to form salts. It is also a weak nucleophile and can react with electrophiles to form addition products. The presence of the chlorine atom on the benzene ring makes it more reactive than acetanilide towards electrophilic aromatic substitution reactions.
Stability and Reactivity
Chloro 2,6-dimethylacetanilide is a relatively stable compound. It is not sensitive to air or moisture and can be stored for long periods of time without decomposing. However, it is incompatible with strong acids and bases and can react violently with oxidizing agents.
Synthesis and Manufacturing
Chloro 2 6 dimethylacetanilide can be synthesized through various methods, each with its advantages and disadvantages. One common approach involves the reaction of 2,6-dimethylaniline with chloroacetyl chloride in the presence of a base such as pyridine. This reaction proceeds via nucleophilic acyl substitution, where the amine group of 2,6-dimethylaniline attacks the carbonyl carbon of chloroacetyl chloride, leading to the formation of the desired product.
Industrial-Scale Manufacturing, A chloro 2 6 dimethylacetanilide
On an industrial scale, chloro 2 6 dimethylacetanilide is typically produced through a multi-step process. The first step involves the nitration of 2,6-dimethylaniline with nitric acid and sulfuric acid, yielding 2,6-dimethyl-4-nitroaniline. This intermediate is then reduced to 2,6-dimethylaniline using iron or other reducing agents.
The resulting 2,6-dimethylaniline is then reacted with chloroacetyl chloride in the presence of a base to obtain chloro 2 6 dimethylacetanilide.
Challenges and Optimizations
The production of chloro 2 6 dimethylacetanilide presents several challenges, including the need for careful control of reaction conditions to avoid the formation of unwanted byproducts. Additionally, the reaction is exothermic, requiring efficient heat management to prevent runaway reactions. Optimizations in the manufacturing process have focused on improving yield, reducing energy consumption, and minimizing waste generation.
Applications and Uses
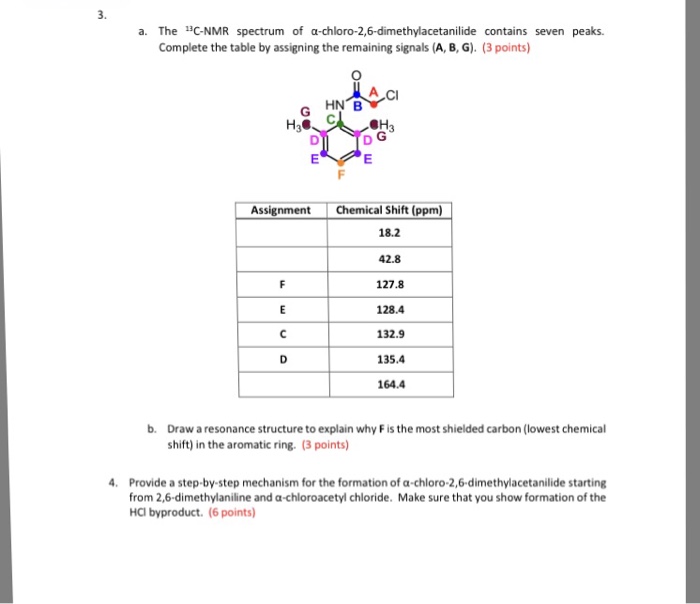
Chloro 2,6-dimethylacetanilide finds applications in diverse industries due to its unique properties. It serves as an intermediate in the production of various chemicals and products, and it is also employed as an additive or component in specific formulations.
Intermediate in Chemical Production
Chloro 2,6-dimethylacetanilide plays a crucial role as an intermediate in the synthesis of other chemicals, including:
- Chloroacetanilides:Chloro 2,6-dimethylacetanilide undergoes reactions to produce other chloroacetanilide derivatives, which are used as intermediates in the manufacture of dyes, pharmaceuticals, and agrochemicals.
- 2,6-Dimethylaniline:It can be reduced to 2,6-dimethylaniline, a key intermediate in the production of dyes, pigments, and antioxidants.
Additive in Formulations
Chloro 2,6-dimethylacetanilide is utilized as an additive or component in various formulations due to its specific properties:
- Fungicide:It exhibits antifungal properties and is incorporated into fungicides to control fungal growth in crops and other applications.
- Antioxidant:Chloro 2,6-dimethylacetanilide possesses antioxidant properties and is added to certain formulations to prevent oxidation and extend product shelf life.
- Stabilizer:It acts as a stabilizer in certain formulations, preventing degradation and maintaining the desired properties over time.
Safety and Regulatory Considerations: A Chloro 2 6 Dimethylacetanilide
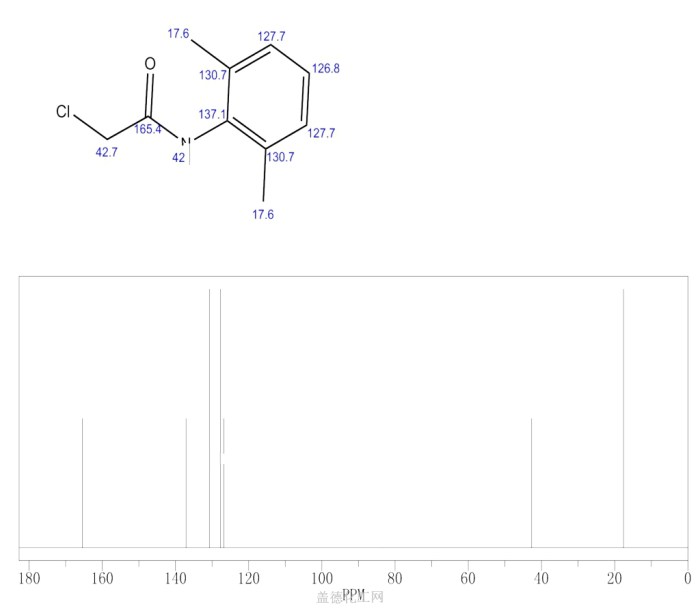
4-Chloro-2,6-dimethylacetanilide (4-chloro-o-toluidine) is a potentially hazardous substance, requiring careful handling and adherence to safety regulations.
Hazards
The primary hazards associated with 4-chloro-2,6-dimethylacetanilide include:
- Acute Toxicity:Inhalation, ingestion, or skin contact can cause irritation, respiratory distress, and systemic toxicity.
- Carcinogenicity:Prolonged exposure has been linked to an increased risk of cancer, particularly bladder cancer.
- Environmental Toxicity:The substance is harmful to aquatic organisms and can accumulate in the environment.
Regulations
To ensure safe handling and minimize risks, regulatory measures are in place for 4-chloro-2,6-dimethylacetanilide:
- Occupational Exposure Limits:Permissible exposure limits (PELs) and threshold limit values (TLVs) have been established to limit workplace exposure.
- Transportation Regulations:The substance is classified as a hazardous material and must be transported in accordance with relevant regulations.
- Environmental Regulations:Discharge into the environment is regulated to minimize ecological impact.
Disposal
Proper disposal of 4-chloro-2,6-dimethylacetanilide is crucial to prevent environmental contamination and health risks:
- Incineration:High-temperature incineration is the preferred method for disposal, ensuring complete destruction.
- Landfilling:Disposal in hazardous waste landfills is acceptable if incineration is not feasible.
- Wastewater Treatment:Treatment in wastewater treatment plants is possible, but requires specialized processes to remove the substance.
Market Analysis and Trends
The global market for chloro 2,6-dimethylacetanilide is experiencing steady growth, driven by increasing demand from various industries. The market is expected to reach a value of approximately USD 1.5 billion by 2027, expanding at a compound annual growth rate (CAGR) of around 4.5% during the forecast period.
Key Market Drivers
- Growing demand from the pharmaceutical industry as an intermediate in the synthesis of various drugs, including anti-inflammatory and analgesic medications.
- Increasing usage in the textile industry as a dye intermediate, particularly in the production of disperse dyes for synthetic fibers.
- Rising demand from the rubber industry as an antioxidant and stabilizer in the production of rubber products, such as tires and hoses.
- Growing adoption in the electronics industry as a component in printed circuit boards (PCBs) and other electronic components.
Challenges
- Environmental regulations and restrictions on the use of certain chemicals, including chloro 2,6-dimethylacetanilide, due to potential toxicity and environmental concerns.
- Availability of substitutes and alternative materials, such as other dyes and antioxidants, which can impact demand for chloro 2,6-dimethylacetanilide.
- Fluctuating raw material prices and supply chain disruptions, which can affect the cost and availability of chloro 2,6-dimethylacetanilide.
Competitive Landscape
The global market for chloro 2,6-dimethylacetanilide is highly competitive, with several major players operating in the industry. Some of the key players include:
- Clariant
- BASF
- Lanxess
- DIC Corporation
- Huntsman
These companies are engaged in ongoing research and development to improve the efficiency and safety of chloro 2,6-dimethylacetanilide production and applications.
Quick FAQs
What are the key applications of a chloro 2 6 dimethylacetanilide?
A chloro 2 6 dimethylacetanilide finds applications as an intermediate in the production of dyes, pharmaceuticals, and other chemicals. It also serves as an additive in plastics, rubber, and coatings to enhance their properties.
What safety precautions should be taken when handling a chloro 2 6 dimethylacetanilide?
A chloro 2 6 dimethylacetanilide can be harmful if ingested, inhaled, or absorbed through the skin. Proper protective gear, including gloves, masks, and protective clothing, should be worn when handling this compound. Adequate ventilation is also essential to prevent exposure to harmful vapors.
What is the market outlook for a chloro 2 6 dimethylacetanilide?
The market for a chloro 2 6 dimethylacetanilide is expected to grow steadily in the coming years, driven by increasing demand from various industries. The compound’s versatility and cost-effectiveness make it an attractive option for manufacturers seeking to enhance the properties of their products.